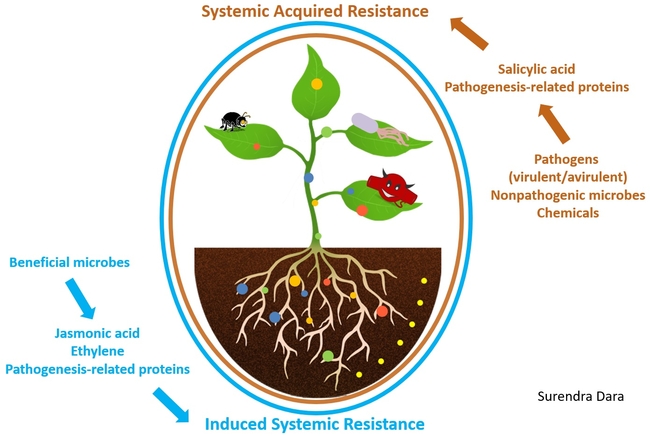
Biostimulants are beneficial microorganisms or substances that can be used in crop production to improve plants' immune responses and their ability to perform well under biotic and abiotic stresses. Biostimulants induce plant resistance to stress factors through systemic acquired resistance or induced systemic resistance. When plants are exposed to virulent and avirulent pathogens, non-pathogenic microorganisms, and some chemicals, the systemic acquired resistance mechanism is activated through the salicylic acid pathway triggering the production of pathogenesis-related proteins. On the other hand, when plants are exposed to beneficial microbes, the induced systemic resistance mechanism is activated through the jasmonic acid and ethylene pathways. The jasmonic acid pathway also leads to pathogenesis-related protein production in plants. In other words, when plants are exposed to pathogens, non-pathogens, or other compounds, various defense genes are activated through two major immune responses, helping plants fight the real infection or prepare them for potential infection. Beneficial microbes and non-microbial biostimulants are like vaccines that prepare plants for potential health problems.
Earlier studies in tomato (Dara and Lewis, 2018; Dara, 2019a) and strawberry (Dara and Peck, 2018; Dara, 2019b) demonstrated varying levels of benefits to crop health and yield improvements from a variety of botanical, microbial, or mineral biostimulants and other supplements. Some of the evaluated products resulted in significant yield improvement in both tomatoes and strawberries compared to the grower standard practices. There are several biostimulant products in the market with a variety of active ingredients, and some also have major plant nutrients such as nitrogen, phosphorus, and potassium. Depending on the crop, growing conditions, potential risk of pests and diseases, and other factors, growers can use one or more of these products. A study was conducted to evaluate the impact of various biostimulants on the yield, quality, and shelf life of strawberries.
Methodology
Strawberry cultivar San Andreas was planted late November 2018 and treatments were administered at the time of planting or soon after, depending on the protocol. Each treatment had a 290' long strawberry bed where 10' of the bed at each end was left out as a buffer. Then, six 30' long plots, each representing a replication, were marked within each bed with an 18' buffer between the plots. Since the test products needed to be applied through the drip system, an entire bed was allocated for each treatment, except for the standard program that had one bed on either side of the experimental block, and plots were marked within each bed for data collection. The following treatment regimens were used in the study:
1. Standard Program (SP): Major nutrients were provided in the form of Urea Ammonium Nitrate Solution 32-0-0, Ammonium Polyphosphate Solution, and Potassium Thiosulfate (KTS 0-0-25). Nitrogen, phosphorus, and potassium were applied before planting in November 2018 at 170, 60, and 130 lb/acre, respectively. From 15 January to 9 May 2019, a total of 26 lb of nitrogen, 13 lb of phosphorus, and 26 lb of potassium were applied through 13 periodic applications.
2. SP + Terramera Program: Formulation labeled as Experimental A (cold-pressed neem 70%) was applied at 1.2% vol/vol immediately after planting. Additional applications were made starting from 2 weeks after planting once every two weeks until the end of February (six times), followed by 13 weekly applications from the beginning of March.
3. SP + Locus Low Rate Program: This program contained Rhizolizer soil amendment (Trichoderma harzianum 1X108 CFU/ml and Bacillus amyloliquefaciens 1X109 CFU/ml) at 3 fl oz/acre, humic acid at 13.5 fl oz/acre, and kelp at 6.8 fl oz/acre. The first application was made within 15 days and at 30 days after planting followed by once in February, March, and April 2019.
4. SP + Locus High Rate Program: This program contained Rhizolizer soil amendment (Trichoderma harzianum 1X108 CFU/ml and Bacillus amyloliquefaciens 1X109 CFU/ml) at 6 fl oz/acre, humic acid at 13.5 fl oz/acre, and kelp at 6.8 fl oz/acre. The first application was made within 15 days and at 30 days after planting followed by once in February, March, and April 2019.
5. SP + BioGro Program: Transplants were treated with Premium Plant BB (Beauveria bassiana 1.1%) by spraying 2 fl oz/acre (1.29 ml in 850 ml of water). About 7 weeks after planting, 30 gpa of Plant-X Rhizo-Pro (botanical extracts), 2 gpa of CHB Premium 21 (humic acid blend), 3 gpa of CHB Premium 6 (3% humic acids), and 5 gpa of NUE Flourish 4-12-0 were applied. Starting from mid-February 2019, 15 gpa of Plant-X Rhizo-Pro, 1 gpa of CHB Premium 21, and 2 gpa of CHB Premium 6 were applied four times every 2 weeks until the end of March. Starting from 5 April 2019, 8 weekly applications of 10 gpa of Plant-X Rhizo-Pro, 1 gpa of CHB Premium 21, 2 gpa of Premium 6, and 4 gpa of NUE Flourish 4-12-0 were made until 26 May 2019.
6. SP + Actagro Program: Structure 7-21-0 at 3 gpa and Liquid Humus 0-0-4 with 22% organic acids at 1 gpa were first applied within 1 week of planting and then three more times every 2 weeks until the end of December 2018. Additional monthly applications were made from the end of January to the end of April 2019.
All the fertilizers and treatment materials were applied through the drip system using the Dosatron (Model D14MZ2) equipment. The following parameters were measured during the experimental period from January to May 2019.
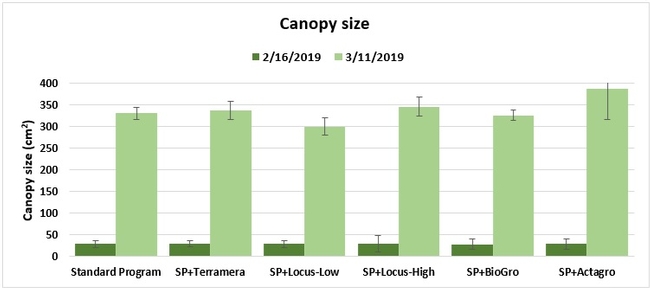
Canopy: The size of the plant canopy was determined on 21 January and again on 17 February 2019 by measuring the spread of the canopy across and along the length of the bed from 16 random plants within each plot, and calculating the area.
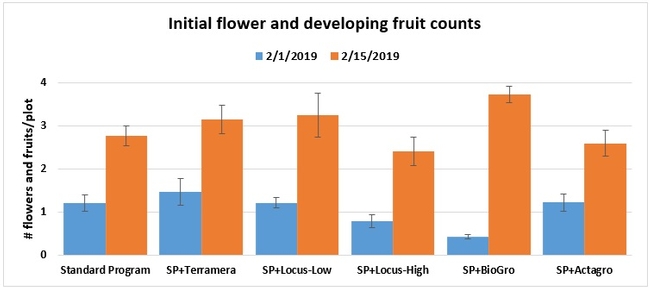
Initial flowering and fruiting: When flowering initiated, the number of flowers and developing fruits was counted from 16 random plants within each plot on 1 and 16 February 2019.
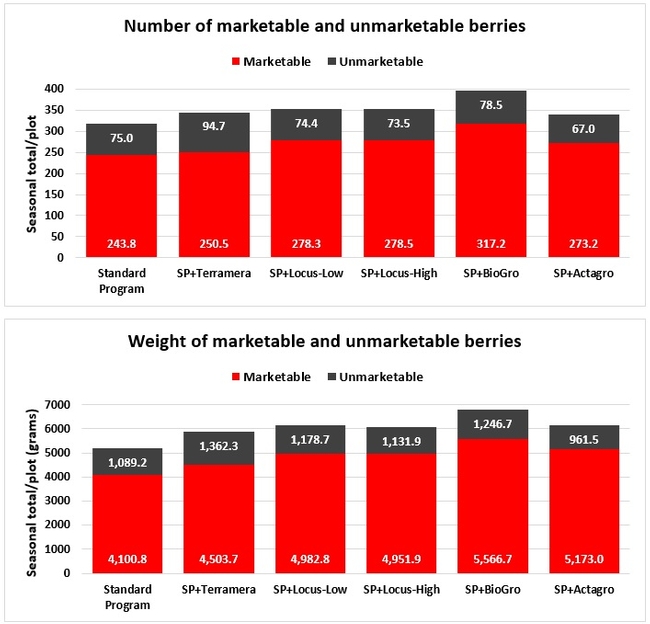
Fruit yield: Fruit was harvested weekly from every plant within each plot from 3 March to 26 May 2019 on 11 dates and the number and weight of the marketable and unmarketable fruit was determined. Due to a technical error, some of the yield data from an additional date (29 March) were lost and excluded from the analysis.
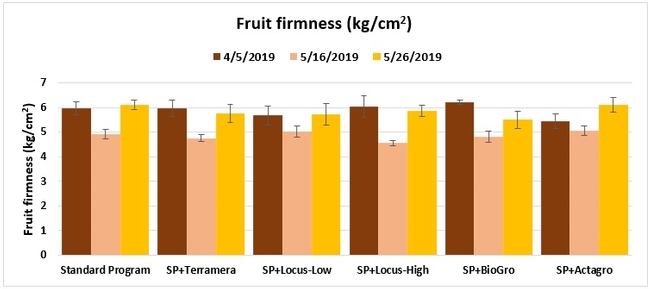
Fruit firmness: The firmness of two marketable fruit from each of five random plants per plot was measured using a penetrometer on 5 April, and 16 and 26 May 2019.
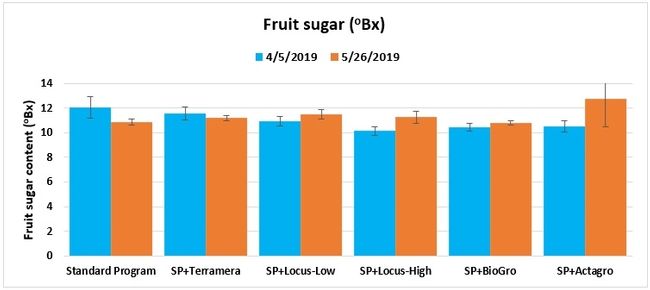
Fruit sugar content: The sugar content from one marketable fruit from each of 10 plants per plot was measured using a refractometer on 5 April and 26 May 2019.
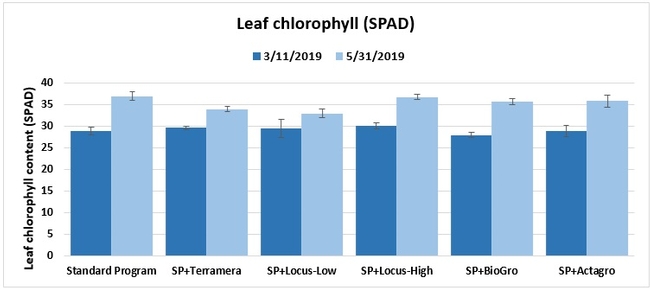
Leaf chlorophyll content: On 11 March and 31 May 2019, the chlorophyll content of one mature leaf from each of five random plants per plot was measured using a chlorophyll meter.
Postharvest disease: Marketable fruit harvested on 21 and 28 April, and 5 and 26 May 2019 was kept at the room temperature in perforated plastic containers (clamshells) and the growth of gray mold (Botrytis cinerea) or Rhizopus fruit rot fungus (Rhizopus spp.) was measured on a scale of 0 to 4 (where 0=no fungus, 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100% fungal growth) 3 and 5 days after each harvest.
Data were analyzed using analysis of variance in Statistix software and significant means were separated using the Least Significant Difference means separation test.
Results and Discussion
Statistically significant differences among treatments were seen for the seasonal total number of unmarketable berries (P = 0.0172), the initial flower and fruit numbers on 1 February (P < 0.0014), the leaf chlorophyll content on 31 May (P = 0.0144), and the disease rating 3 days after the 28 April harvest (P = 0.0065).
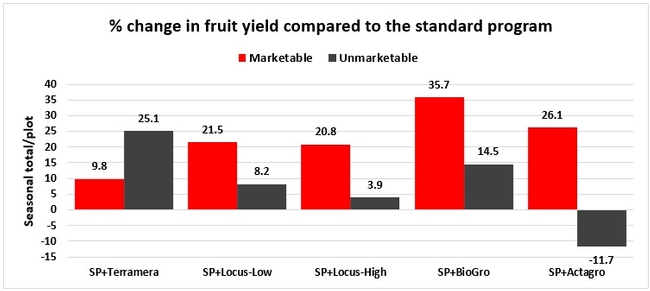
Treatments did not differ (P > 0.05) in any other measured parameters of the plant, fruit quality, or yield. However, the total seasonal fruit yield was 13 to 31% higher and the total marketable fruit yield was 10 to 36% higher in various treatment programs compared to the standard program. The seasonal total of unmarketable fruit yield was also 4 to 25% higher in treatment programs than the standard program except that there were nearly 12% fewer unmarketable berries in the Actagro program compared to the standard program.
While treatments did not statistically differ for many of the measured parameters, numerical differences in marketable fruit yield could be helpful for some understanding of the potential of these biostimulants. Additional studies with larger treatment plots would be useful for generating additional data.
Acknowledgments
Thanks to Dr. Jenita Thinakaran for the assistance at the start of the study, Hamza Khairi for his technical assistance throughout the study, the field staff at the Shafter Research Station for the crop maintenance, NorCal Nursery for the strawberry transplants, and Actagro, BioGro, Locus, and Terramera for their collaboration and financial support
References
Dara, S. K. 2019a. Improving tomato yield with nutrient materials containing microbial and botanical biostimulants. eJournal of Entomology and Biologicals, 6 June 2019 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=30448
Dara, S. K. 2019b. Evaluating the efficacy of anti-stress supplements on strawberry yield and quality. eJournal of Entomology and Biologicals, 10 August 2019 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=31044
Dara, S. K. and D. Peck. 2018. Microbial and bioactive soil amendments for improving strawberry crop growth, health, and fruit yields: a 2017-2018 study eJournal of Entomology and Biologicals, 3 August 2018 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=27891
Dara, S. K. and E. Lewis. 2018. Impact of nutrient and biostimulant materials on tomato crop health and yield. eJournal of Entomology and Biologicals, 9 January 2019 https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=26054