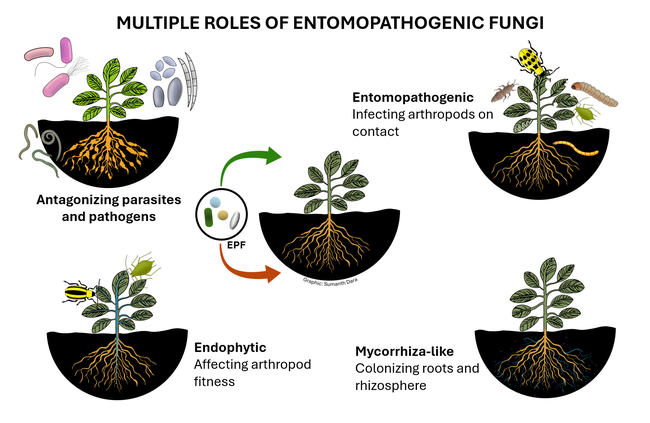
Entomopathogenic fungi (EPF) are those that infect various arthropods such as ticks, mites, and insects. There are two major groups of EPF that play an important role in pest suppression. Members of the order Entomophthorales are more host-specific and examples include Entomophaga maimaiga in spongy moth, Entomophthora muscae and Strongwellsea spp. in flies, Conidiobolus obscurus, Entomophthora planchoniana, Neozygites fresenii and Pandora neoaphidis in aphids, and Neozygites floridana in mites. These naturally occurring EPF are fastidious and cannot be mass produced on a commercial scale, but cause epizootics when host populations are high and environmental conditions are favorable resulting in significant pest suppression. On the other hand, members of the order Hypocreales are more generalistic pathogens and can be infective to a variety of arthropods. Beauveria bassiana, Cordyceps fumosorosea, Hirsutella thompsonii, and Metarhizium brunneum are some examples of hypocrealeans. These can be grown on artificial media on a commercial scale and several biopesticide formulations based on various isolates of these fungi are available in the US and elsewhere. Both entomophthoralean and hypocrealean fungi have the same mode of infection. When fungal spores come in contact with their host, they germinate and enter the host body through mechanical pressure and enzymatic degradation of the cuticle. They multiply inside the host, invade the tissues, and finally emerge from the cuticle to produce spores that continue the infection process.
With growing emphasis on sustainable crop production with safer pesticides, the market for biopesticides including EPF-based ones has been increasing. Newer EPF isolates and modern technology contributed to the development of improved formulations. EPF-based products can be used for soil-inhabiting pests or their life stages like root aphids, pupae of thrips, and wireworms to foliar feeders or above-ground pests including the members of Coleoptera, Diptera, Hemiptera, Orthoptera, Thysanoptera, and others. Considering their potential against a variety of pests on multiple crops, EPF-based pesticides should be an important part of integrated pest management (IPM) programs. However, there is a significant knowledge gap in effectively using EPF in IPM and fully exploring their potential in sustainable crop production.
Since EPF spores need to come in contact with the host, using them against the right pest or life stage is very important to obtain desired results. Sometimes, using EPF in combination or rotation with botanical or synthetic pesticides is more effective than using them alone against a particular pest (Dara 2013; 2015; 2016). As EPF formulations contain live fungi, label instructions should be followed for proper storage, transportation, tank-mixing, and application to maintain their efficacy. Compatibility can vary according to the EPF and its formulation, but studies showed that some isolates of Beauveria bassiana and Metarhizium anisopliae are compatible with several fungicides (Dara, et al., 2014; Roberti et al., 2017; Khun et al., 2021).
In addition to controlling arthropod pests, EPF being soilborne fungi also have a direct relationship with plants and other microbes. EPF colonize plant tissues and grow inside the plants in a phenomenon known as endophytism. Endophytic EPF grow as hyphae and do not produce spores. Although they cannot cause infection to pests feeding on those plants, they indirectly affect pests by reducing their fitness and survival by activating induced systemic resistance. When EPF are applied to soil, they form a mycorrhiza-like relationship with plant roots and help plants withstand biotic stresses and improve nutrient uptake. EPF can also antagonize plant pathogens through competitive displacement and antimicrobial activity. Thus soil and foliar application of EPF-based pesticides result in additional benefits in improving crop growth and health in addition to controlling pests through infection.
Several studies explored the non-entomopathogenic roles of EPF (Dara, 2019a). Soil application of B. bassiana had a positive impact on the survival, growth, and health of cabbage plants growing under water stress (Dara et al., 2017). Metarhizium brunneum also had a similar impact on plant growth in this study. Root and rhizosphere colonization by Metarhizium spp.improved shoot length and root weight in industrial hemp (Hu et al., 2023) and root colonization of Metarhizium robertsii alleviated hemp from salt and drought stress. Metarhizium spp. and B. bassiana transferred nitrogen from dead insects to the plant they colonized (Behie et al., 2012; Behie and Bidochka, 2014). These studies show the role of EPF in soil nitrogen cycle and how plants benefit from the endophytic relationship of EPF. Additionally, recent reports showed that endophytic B. bassiana induced the biosynthesis of flavonoids in oilseed rape (Muola et al., 2023) and flavonol content in licorice plants (Etsassala et al., 2023).
Seed treatment with B. bassiana increased plant height, stem diameter, number of leaves, shoots and apical buds, biomass, and total chlorophyll content in cotton and reduced cotton aphid (Aphis gossypii) populations (Mantzoukas et al., 2023). Similarly, endophytic B. bassiana significantly reduced the reproductive rate and populations of the Russian wheat aphid (Diuraphis noxia) in South African wheat (Motholo et al., 2023). In corn, endophytic B. bassiana and M. anisopliae negatively impacted the survival, development, and reproduction of the fall armyworm (Spodoptera frugiperda) (Altaf et al., 2023).
Soil application of B. bassiana, Cordyceps fumosorosea, and Metarhizium brunneum antagonized Fusarium oxysporum f.sp. vasinfectum in cotton as effectively as some biofungicides (Dara et al., 2020). Beauveria bassiana treatment at a higher rate provided significantly better protection than all other treatments in this study. Both B. bassiana and C. fumosorosea inhibited the growth of F. oxysporum in vitro (Yanagawa et al., 2021). In corn, endophytic M. robertsii promoted plant growth and reduced southern corn leaf blight caused by Cochliobolus heterostrophus (Imtiaz et al., 2023). Induced systemic resistance is thought to be responsible for this protection. Similarly, B. bassiana applied as seed treatment, seedling root dip, and foliar spray reduced the incidence of rice sheath blight caused by Rhizoctonia solani by 69% and its severity by 60% under field conditions (Deb et al., 2023). Beauveria bassiana also resulted in 71% of mycelial inhibition in R. solani through the production of cell wall degrading enzymes, release of secondary metabolites, and mycoparasitism.
Multiple recent studies showed that EPF also have a negative impact on plant-parasitic nematodes. Beauveria bassiana and C. fumosorosea reduced the survival ofthe root-knot nematode, Meloidogyne incognita, in vitro (Yanagawa et al., 2021). Similar to the nematophagous fungus Purpureocillium lilacinum, both B. bassiana and M. anisopliae were effective in reducing galls caused by M. incognita in tomato and cucumber (Karabörklü et al., 2022). Metarhizium anisopliae was as effective as P. lilacinum with 75% reduction in gall formation and 85% control of second instar juveniles in tomato. Beauveria bassiana and M. anisopliae also resulted in about 85% control of second instar juveniles in cucumber. In another study, soil application of B. bassiana significantly reduced nematode infestation in tomato roots and B. bassiana treatment caused 60% mortality in nematodes in a lab assay (Kim et al., 2023). Volatile organic compounds, 1-octen-3-ol and 3-octanone from M. brunneum attracted and killed another plant-parasitic nematode, Meloidogyne hapla, in lab assays (Khoja et al., 2021).
As many of these recent studies indicated, the non-entomopathogenic roles of EPF is a new area of applied research interest with tremendous practical benefits. In addition to direct pest control through infection, EPF as endophytes offer multiple benefits in suppressing pest populations by affecting their fitness, antagonizing plant pathogens and plant-parasitic nematodes, imparting drought and salt tolerance in plants, improving nutrient uptake, and promoting overall growth and health of plants. Using EPF-based biopesticides comes under the microbial control of IPM (Dara, 2019b) and will contribute to insecticide resistance management. Additionally, the non-target benefits of EPF will help growers optimize the use of other inputs and related costs. EPF can be very important in sustainable crop production and a thorough understanding of their biology, interactions with pests, plants, pathogens, and other biotic and abiotic factors, and effective use strategies will help achieve their full potential.
Note: This article was initially published in the December 2023 issue of CAPCA Adviser magazine.
References
Altaf, N., M. I. Ullah, M. Afzal, M. Arshad, S. Ali, M. Rizwan, L. A. Al-Shuraym, S. S. Alhelaify, and S. Sayed. 2023. Endophytic colonization by Beauveria bassiana and Metarhizium anisopliae in maize plants affects the fitness of Spodoptera frugiperda (Lepidoptera: Noctuidae). Microorganisms 11: 1067.
Behie, S. W. and M. J. Bidochka. 2014. Ubiquity of insect-derived nitrogen transfer to plants by endophytic insect-pathogenic fungi: an additional branch of the soil nitrogen cycle. Appl. Environ. Microbiol. 80: 1553-1560.
Behie, S. W., P. M. Zelisko, and M. J. Bidochka. 2012. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 336: 1576-1577.
Dara, S. 2013. Microbial control as an important component of strawberry IPM. CAPCA Adviser, 16 (1): 29-32.
Dara, S. K. 2015. Root aphids and their management in organic celery. CAPCA Adviser 18 (5): 65-70.
Dara, S. K. 2016. IPM solutions for insect pests in California strawberries: efficacy of botanical, chemical, mechanical, and microbial options. CAPCA Adviser 19 (2): 40-46.
Dara, S. K. 2019a. Non-entomopathogenic roles of entomopathogenic fungi in promoting plant health and growth. Insects 10: 277.
Dara, S. K. 2019b. The new integrated pest management paradigm for the modern age. JIPM 10: 12.
Dara, S. K., S. S. Dara, and S.S.R. Dara. 2020. Managing Fusarium oxysporum f. sp. vasinfectum Race 4 with beneficial microorganisms including entomopathogenic fungi. Acta Horticulturae 1270: 111-116.
Dara, S. K., S.S.R. Dara, and S. S. Dara. 2017. Impact of entomopathogenic fungi on the growth, development, and health of cabbage growing under water stress. Am. J. Plant Sci. 8: 1224-1233.
Dara, S.S.R., S.S. Dara, A. Sahoo, H. Bellam, and S. K. Dara. 2014. Can entomopathogenic fungus Beauveria bassiana be used for pest management when fungicides are used for disease management? UCANR eJournal of Entomology and Biologicals October 23, 2014.
Deb, L., P. Dutta, M. K. Mandal and S. B. Singh. 2023. Antimicrobial traits of Beauveria bassiana against Rhizoctonia solani, the causal agent of sheath blight of rice under filed conditions. Plant Disease PDIS-04. https://doi.org/10.1094/PDIS-04-22-0806-RE.
Etsassala, N. G. E. R., N. Macuphe, I. Rhoda, F. Rautenbach and F. Nchu. 2023. An endophytic Beauveria bassiana (Hypocreales) strain enhances the flavonol contents of Helichrysum petiolare. In Sustainable Uses and Prospects of Medicinal Plants, eds. L. Kambizi and C Bvenura, CRC Press. pp 367-377.
Hu, S. and M. J. Bidochka. 2023. Colonization of hemp by Metarhizium and alleviation of salt and drought stress. 55th Annual meetings of the Society for Invertebrate Pathology, July 30-August 3, 2023, College Park, MD, pp. 56-57.
Hu, S., M. S. Mojahid, M. J. Bidochka. 2023. Root colonization of industrial hemp (Cannabis sativa L.) by the endophytic fungi Metarhizium and Pochonia improves growth. Industrial Crops and Products 198: 116716.
Imtiaz, A. M. M. Jiménez-Gasco, and M. E. Barbercheck. 2023. Endophytic Metarhizium robertsii suppresses the phytopathogen, Cochliobolus heterostrophus and modulates maize defenses. 55th Annual meetings of the Society for Invertebrate Pathology, July 30-August 3, 2023, College Park, MD, pp. 66-67.
Karabörklü, S., V. Aydinli and O. Dura. 2022. The potential of Beauveria bassiana and Metarhizium anisopliae in controlling the root-knot nematode Meloidogyne incognita in tomato and cucumber. J. Asia-Pacific Entomol. 25: 101846.
Khoja, S., K. M. Eltayef, I. Baxter, A. Myrta, J. C. Bull and T. Butt. 2021. Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol. Con. 152: 104472.
Khun, K. K., G. J. Ash, M. M. Stevens, R. K. Huwer, B. A. Wilson. 2021. Compatibility of Metarhizium anisopliae and Beauveria bassiana with insecticides and fungicides used in macadamia production in Australia. Pest Manag. Sci. 77: 709-718.
Kim, K. J., S. E. Park, Y. Im, H. Yang and J. S. Kim. 2023. Drenching of Beauveria bassiana JEF-503 reduces the root knot nematode populations in soil. 55th Annual meetings of the Society for Invertebrate Pathology, July 30-August 3, 2023, College Park, MD, pp. 68.
Mantzoukas, S., V. Papantzikos, S. Katsogiannou, A. Papanikou, C. Koukidis, D. Servis, P. Eliopoulos, and G. Patakioutas. 2023. Biostimulant and bioinsecticidal effect of coating cotton seeds with endophytic Beauveria bassiana in semi-field conditions. Microorganisms 11: 2050.
Motholo, L. F., M. Booyse, J. L. Hatting, T. J. Tsilo, M. Lekhooa, and O. Thekisoe. 2023. Endophytic effect of the South African Beuaveria bassiana strain PPRI 7598 on the population growth and development of the Russian wheat aphid, Diuraphis noxia. Agriculture 13: 1060.
Moula, A., T. Birge, M. Helander, S. Mathew, V. Harazinova, K. Saikkonen, and B. Fuchs. 2023. Endophytic Beauveria bassiana induces biosynthesis of flavonoids in oilseed rape following both seed inoculation and natural colonization. Pest Manag. Sci. DOI 10.1002/ps.7672
Roberti, R. H. RIghini, A. Masetti, and S. Maini. 2017. Compatibility of Beauveria bassiana with fungicides in vitro and on zucchini plants infested with Trialeurodes vaporariorum. Biol. Con. 113: 39-44.
Yanagawa, A., N.P.R.A. Krishanti, A. Sugiyama, E. Chrysanti, S. K. Ragamustari, M. Kubo, C. Furumizu, S. Sawa, S. K. Dara, and M. Kobayashi. 2022. Control of Fusarium and nematodes by entomopathogenic fungi for organic production of Zingiber officinale. J. Natural Medicines, 76: 291-297.