Eggs, nymphs, and adult silverleaf whitefly on zucchini. Photo by Surendra Dara
A study was conducted in the summer of 2017 to evaluate the efficacy of various chemical, botanical, and microbial pesticides against arthropod pests on zucchini. Zucchini plants initially had a high aphid infestation, but populations gradually declined due to natural control by lady beetle activity. However, heavy silverleaf whitefly (Bemisia tabaci) infestations developed by the time the study was initiated. Other pests that were present during the study period were aphids (possibly melon aphids), the western flower thrips (Frankliniella occidentalis), and the pacific spider mite (Tetranychus pacificus).
Pacific spider mite (egg, male, and females), western flower thrips larva, and unknown aphids on zucchini. Photo by Surendra Dara
Methodology
Experiment was conducted using a randomized complete block design with 10 treatments. Each treatment had two 38” wide and 300' long rows of zucchini replicated four times. Treatments included i) untreated control, ii) Sivanto 200 SL (flupyradifurone) 14 fl oz/ac, iii) Sequoia (sulfoxaflor) 2.5 fl oz/ac, iv) Venerate XC (heat-killed bacterium, Burkholderia rinojensis strain A396) 4 qrt/ac, v) PFR-97 20% WDG (entomopathogenic fungus, Isaria fumosorosea Apopka strain 97) 2 lb/ac, vi) I1800AA (undisclosed botanical extract) 10.3 fl oz/ac, vii) I1800A 12.7 fl oz, viii) I1800A 17.1 fl oz, ix) I1800A 20.5 fl oz, and x) VST-00634LC (based on a peptide in spider venom) 25%. A spray volume of 50 gpa for all treatments except for VST-00634LC, which had 25 gpa. Treatments were applied on 28 August and 4 September, 2017 using a tractor-mounted sprayer with three Teeject 8003vs flat spry nozzles that covered the top and both sides of each bed.
Pest populations were counted before the first spray application and 4 days after each application. On each sampling date, one mid-tier leaf was collected from each of the five randomly selected plants within each plot. A 2-square inch disc was cut out from the middle of each leaf and the number of aphids, eggs and nymphs of silverleaf whitefly, larvae of western flower thrips, and eggs and mobile stages of pacific spider mite were counted under a dissecting microscope. Data were analyzed using Statistix software and Tukey's HSD test was used to separate significant means.
Spraying, sampling, and counting
Results
Efficacy varied among different treatments and for different pests.
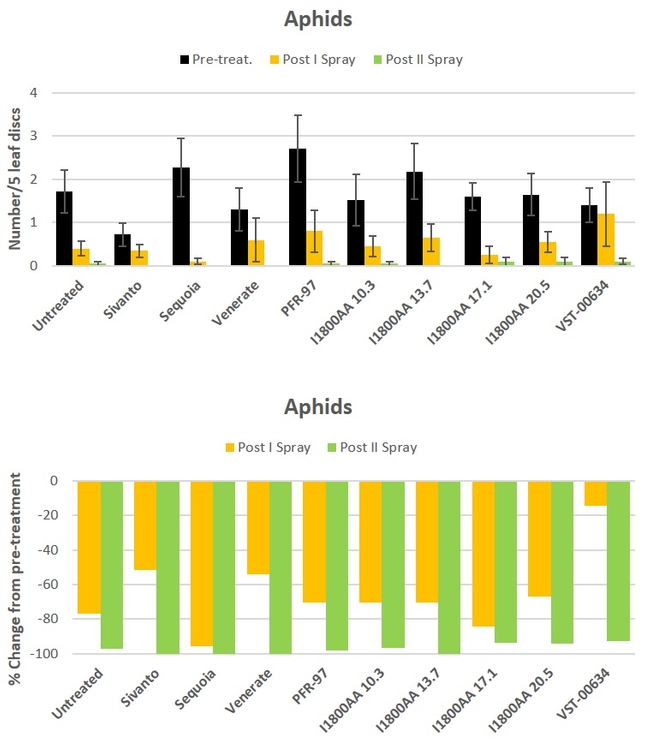
Aphid: There was a general decline in aphid populations during the study period and there was no difference (P > 0.05) among the treatments (Fig. 1).
Fig. 1. Aphid numbers and percent change from pre-treatment counts
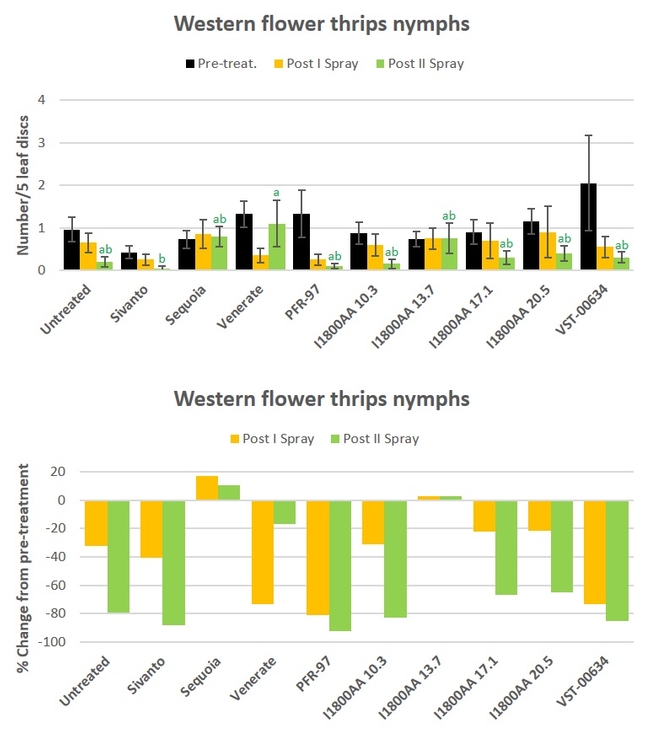
Western flower thrips: Nymphal numbers declined in most of the treatments during the observation period (Fig. 2). However, significant differences (P = 0.0220) only after the second spray application where Sivanto treatment had significantly fewer thrips than Venerate treatment (Fig. 2). There was a 92.5% decline by the end of the study, compared to the pre-treatment counts, from PFR-97 application, followed by 88.1% decline in Sivanto, 85.4% in VST-00634, and 82.9% in I1800AA at 10.3 fl oz.
Fig. 2. Western flower thrips larvae and percent change from pre-treatment counts
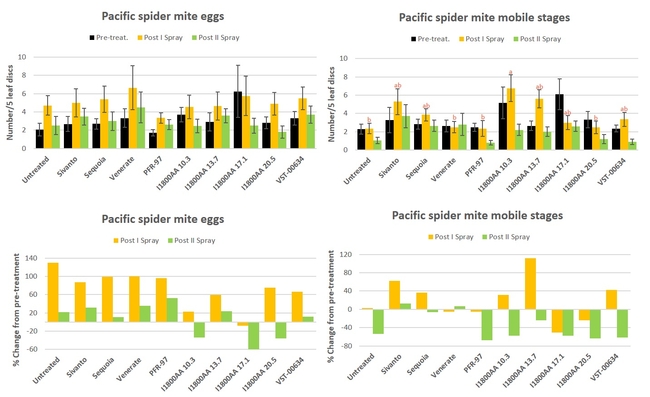
Pacific spider mite: There was an increase in mite eggs in all treatments after the first spray application followed by a decline after the second one without significant differences (P > 0.05) (Fig. 3) Similar trend was also seen in mobile stages in some treatments. Number of mobile stages was significantly different (P = 0.0025) only after the first spray where untreated control, PFR-97, Venerate, and I1800AA at 20.5 fl oz had the lowest. When percent change in egg numbers from the pre-treatment counts, only I1800AA treatments reduced egg numbers after the second spray with a 33.8% decline at 10.3 fl oz rate, 35.7% at 20.5 fl oz, and 60% at 17.1 fl oz. There was also a decline in the mobile stages after the second spray with 54.1% reduction in untreated control to 67.7% in PFR-97 treatment.
Fig. 3. Pacific spider mite egg and mobile stages and percent change from pre-treatment counts
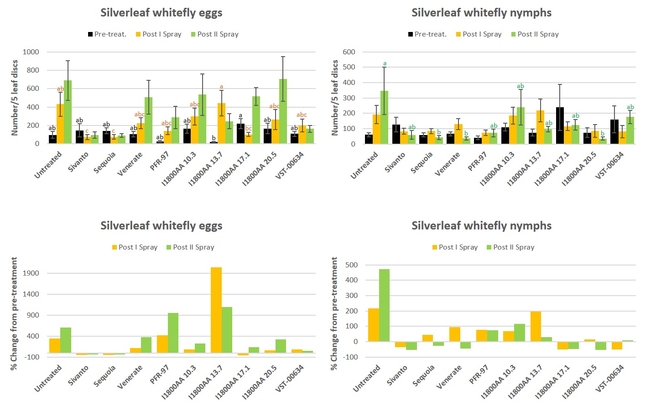
Silverleaf whitefly: There was a general increase in the egg and nymphal stages of whitefly during the study (Fig. 4). Significant differences were seen pre-treatment counts of egg (P = 0.0330) and nymphal stages (P = 0.0011), and after the second spray in nymphal stages (P = 0.0220). Compared to the untreated control, both Sivanto and Sequoia resulted in a significant reduction in egg numbers after the first spray, whereas Sequoia, Venerate, and I1800AA at 20.5 fl oz reduced nymphal stages after the second spray. When the percent change from the pre-treatment counts was compared, only Sivanto and Sequoia reduced whitefly egg numbers after both sprays. There was also a reduction in eggs after the first spray from I1800AA at 17.1 fl oz. However, there was a reduction in nymphal stages after the first spray in Sivanto, I1800AA at 17.1 fl oz, and VST-00634, and after the second spray in Sivanto, Sequoia, Venerate, and I1800AA at 17.1 and 20.5 fl oz.
Fig. 4. Silverleaf whitefly egg and nymphal stages and percent change from pre-treatment counts
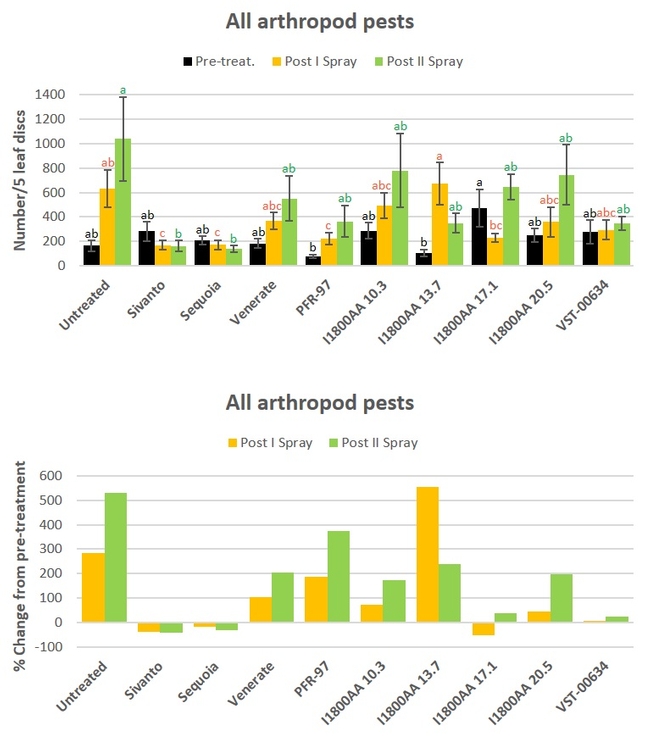
All arthropod pests: When all data were combined for different pests and their life stages, Sivato, Sequia, and PF-97 resulted in a significant (P = 0.0001) decline in pest numbers compared to untreated control after the first spray. Only Sivanto and Sequoia caused such a reduction (P = 0.0048) after the second spray.
Fig. 5. All arthropod pest numbers and percent change from pre-treatment counts
In general, both the chemical pesticides (Sivanto and Sequoia) provided a very good pest control. The efficacy of the botanical extract was moderate to good depending on the pest, life stage, or the application date. Spider venom-based product also provided a good control while microbial products had a moderate impact. Although chemical pesticides appeared to be very efficacious, non-chemical alternatives were also effective. It is important to consider all these options to apply in combinations or rotations to obtain desired pest suppression without posing the risk of insecticide resistance.
Acknowledgements: Thanks for the financial support of Arysta LifeScience, CertisUSA, Dow AgroSciences, and Vestaron, and the technical assistance of Neal Hudson.