The western Grapeleaf skeletonizer (WGLS), Harrisina metallica Stretch (Lepidoptera: Zygaenidae), previously known to cause severe defoliation to vineyards and backyard grapevines appears to be re-emerging in California. Since its first detection in San Diego in 1941, WGLS spread through commercial vineyards and backyard grapes becoming a serious problem. Although two biological control agents from Arizona and Mexico were introduced in California for WGLS control, a naturally occurring granulovirus (Harrisina brillians granulovirus) nearly eradicated WGLS populations and kept them under control. WGLS has not been a problem especially in conventional vineyards. However, based on some unpublished observations, WGLS populations are emerging in organic vineyards and backyard grapevines.
WGLS lives up to its name by skeletonizing and defoliating grape leaves. Organic vineyards are especially at risk and uncontrolled populations can destroy vineyards resulting in significant losses. Metallic bluish or greenish black moths lay barrel shaped yellowish eggs on the lower side of the leaves. There are five larval instars. Early instars are cream colored and develop black and purple bands in later stages. Pupation occurs in a whitish cocoon. Upon hatching, larvae start feeding side by side in a row on the lower side of leaf. Damage by younger larvae appears as whitish leaf area containing veins and the upper cuticle, which eventually turn brown. Older larvae skeletonize leaves leaving larger veins. Larvae may also feed on fruit leading to bunch rot. Severe damage can cause defoliation and sunburn of the exposed fruit.
Methodology
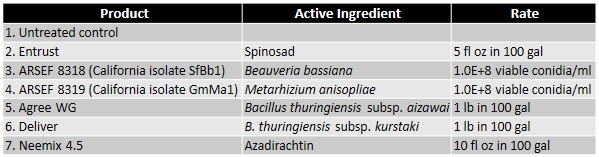
A study was conducted to evaluate the efficacy of six non-chemical control options that included formulations of spinosad, two subspecies of Bacillus thuringiensis, and a botanical insecticide/growth regulator along with two unformulated entomopathogenic fungal isolates native to California. Larvae were collected from an infested, untreated backyard grapevine and maintained in one gallon plastic tubs with screened lids on infested leaves. Fresh, untreated grape leaves from uninfested vines were provided daily for 3 days before starting the assay. For each treatment, five 4-5 instar larvae were placed on a grape leaf disc (rinsed in water and dried) in a Petri plate (100 mm dia) with a moist filter paper. Larvae were treated by spraying 1 ml of the treatment solution (containing Dyne-Amic as a surfactant at 0.125% vol/vol). Application rates for commercial formulations were determined based on label recommendations for 100 gallons of spray volume. Entomopathogenic fungal concentrations were also determined based on the label rates for similar commercial products. Treatments were replicated four times and the assay was conducted twice. Larval mortality was observed daily and dead larvae were removed and incubated separately. Fresh leaf discs were provided as needed to the remaining larvae. Actual and corrected (for control mortality) total mortality were calculated.Data were arcsine-transformed for statistical analysis and significant means were separated using Tukey's HSD test.
Results
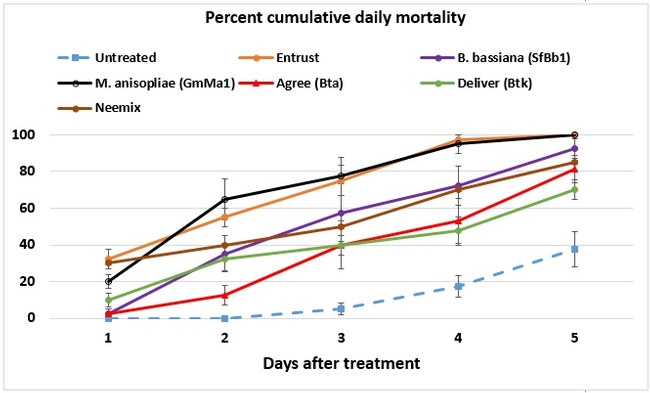
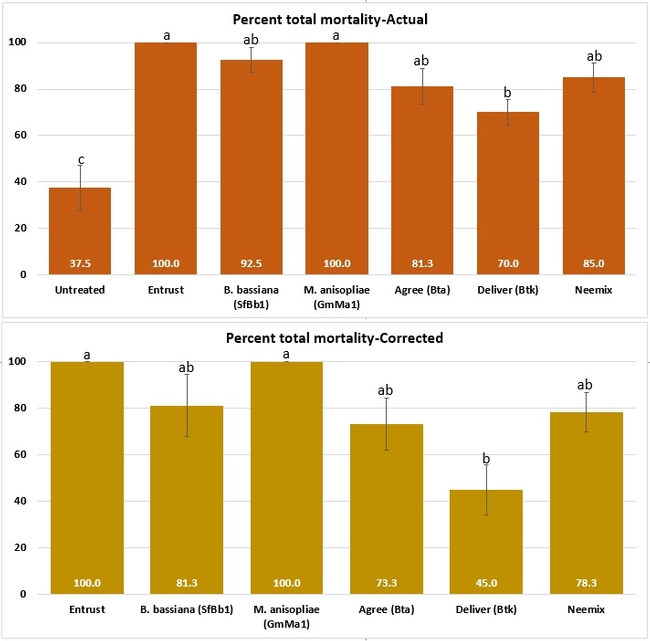
Both cumulative daily mortality and total mortality significantly (P < 0.0001) differed among treatments. Entrust and M. anisopliae resulted in the highest mortality followed by B. bassiana, Neemix, and Agree. In general, feeding reduced or ceased in all larvae following treatment and could have contributed to a lower mortality in B. thuringiensis treatments. Entomopathogenic fungi emerged from all the cadavers from respective treatments. Microbial and botanical options provided good control of WGLS. These non-chemical alternatives can be effectively used in both organic and conventional vineyards. California isolates of B. bassiana and M. anisopliae demonstrated good control efficacy and the potential to be developed as microbial pesticides.
Acknowledgements: Thanks to the technical assistance of Alor Sahoo in carrying out these assays, and Certis and Corteva for providing the pesticide formulations.
References
Federici, B. A. and V. M. Stern. 1990. Replication and occlusion of a granulosis virus in larval and adult midgut epithelium of the western grapeleaf skeletonizer, Harrisina brillians. J. Invertebr. Pathol. 56: 401-414.