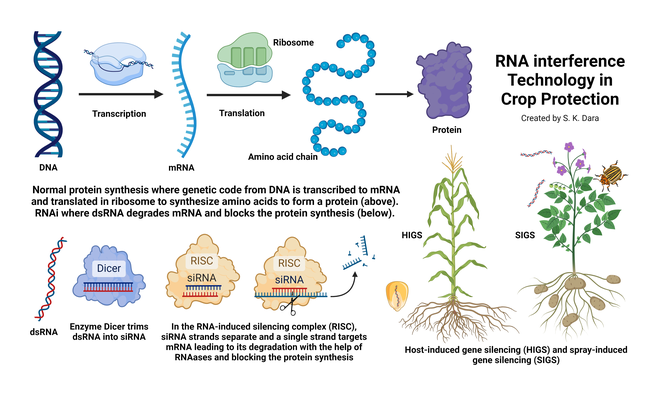
As the food production faces the persistent threat of endemic and invasive pests, researchers continue to develop new technologies and strategies for protecting crops from these threats. One such new technology is RNA interference (RNAi) with targeted mechanisms towards specific pests. RNAi can be used as a trait in a crop or as a sprayable product against the target pest. Before delving further into this here are a few basic details of this biological process that will help understand the RNAi mechanism.
Deoxyribonucleic acid (DNA) in the chromosomes of most living organisms contains genetic code for making proteins that are essential for various biological processes. Ribonucleic acid (RNA) carries the genetic code from DNA to the protein-making factories within the cell known as ribosomes. DNA has two strands of nucleotides (sets of deoxyribose sugar with nitrogenous bases connected by a phosphate group) whereas RNA has only one strand of nucleotides. RNA also differs from DNA in having ribose sugar, instead of deoxyribose, and a different kind of nitrogenous base. The purpose of RNA is to transfer the genetic code from DNA as amino acids are made in ribosomes. A chain of amino acids makes a specific protein. Examples of proteins in insects include juvenile hormone responsible for development and reproductive maturation, ecdysone responsible for molting and metamorphosis, digestive enzymes like amylases, glycosidases, lipases, and proteases, and esterases that are important in metabolizing various compounds that regulate behavior, development, insecticidal resistance, and other processes.
RNAi involves silencing the expression of a specific gene by double-stranded RNA (dsRNA) pieces (either small interfering RNA or microRNA each containing about 21-23 nucleotide pairs) attaching to messenger RNA (mRNA) carrying the code from DNA and thus interfering with the production of a specific protein. RNAi is also known as post-transcriptional gene silencing because the silencing is done after the DNA code is transcribed to mRNA. RNAi is a natural phenomenon that helps organisms to defend against infections or regulate gene expression. For example, when there is a viral infection, cells activate RNAi to destroy virus particles. RNAi-based therapies are currently used in the medical field to treat cancer and neurological issues and to regulate oxalic acid in urine or the low-density lipoprotein cholesterol in blood.
RNAi can be used in agriculture for improving yield or quality, imparting abiotic stress tolerance or pest resistance, and incorporating other desirable traits or as biopesticides in crop protection (Bharathi et al., 2023; Chaudhary et al., 2024). Many research studies have been exploring the RNAi potential in agriculture for decades (Fletcher et al., 2020). Modifying plant height in apple (Zhao et al., 2016), rice (Qiao et al., 2007), and tomato (Cheng et al., 202); imparting drought, salt, and heat tolerance in cotton (Abdurakhmonov et al., 2014), abiotic stress tolerance in cereal crops (Dubrovna et al., 2023), and cold tolerance in tomato (Jiao et al., 2024); imparting resistance to blast (Magnaporthe grisea) and leaf blight (Xanthomonas oryzae pv. oryzae) in rice (Jiang et al., 2009), citrus canker (Xanthomonas citri subsp. citri) in citrus (Enrique et al., 2011), late blight (Phytophthora infestans) in potato (Eschen-Lippold et al., 2012), Fusarium head and seedling blight (Fusarium graminearum) in wheat (Cheng et al., 2015), soybean mosaic virus in soybean (Kim et al., 2016); imparting resistance to bollworm (Helicoverpa armigera) in cotton (Mao et al., 2007 and 2011) and resistance to brown planthopper (Nilaparvata lugens) in rice (Zha et al., 2011); and imparting resistance to root-knot nematode (Meloidogyne incognita) in tomato (Dutta et al., 2015) and soybean cyst nematode (Heterodera glycines) in soybean (Guo et al., 2015) are some of the examples of improving crop traits.
The first RNAi crop in the United States is corn (SmartStax® PRO) against the western corn rootworm (Diabrotica virgifera virgifera) containing both Bacillus thuringiensis toxins and RNAi technology (Head et al., 2017). With its ability to resist both below- and above-ground lepidopteran pests, this hybrid is an important IPM tool. This hybrid is also available in Canada for cultivation, and grain and products from the hybrid are approved for consumption in the European Union. RNAi-based crops are not considered genetically modified organisms (GMOs) because they do not contain a foreign gene to express a particular protein like GMOs but use a natural mechanism to silence a particular gene.
In addition to adding desirable traits to crops, RNAi has also been explored or developed for treating plants against pests and diseases. While RNAi crops use the host-induced gene silencing (HIGS) method, RNAi biopesticides use the spray-induced gene silencing (SIGS). SIGS has been explored for controlling Fusarium graminearum in barley (Koch et al., 2016), sucking and/or stem-boring insects in multiple crops (Li et al. 2015; Hunter and Wintermantel, 2021; Jain et al., 2022), hawthorn spider mite (Amphitetranychus viennensis) in fruit trees and woody ornamentals (Yang et al., 2023). The first sprayable formulation of RNAi-based biopesticide is CalanthaTM from GreenLight Biosciences against the Colorado potato beetle (CPB), Leptinotarsa decemlineata (Rodrigues et al., 2021). The active ingredient is a dsRNA molecule known as Ledprona (Leptinotarsa decemlineata-specific recombinant double-stranded interfering Oligonucleotide GS2). It belongs to a new class of insecticides under group 35 as an RNAi-mediated target suppressor. Applied as a foliar spray, Ledprona suppresses the gene that produces proteasome subunit beta type-5 (PSBT5) in CPB and arrests insect feeding within 2-3 days after it is ingested leading to the death of the pest. PSBT5 is an essential protein important in maintaining cellular protein quality by degrading damaged or misfolded proteins or proteins that are no longer needed.
RNAi can also be used to protect honey bees from the Israeli Acute Paralysis Virus (Hunter et al., 2010) and the Varroa mite (Garbian et al., 2012). In field studies, honey bee populations and honey production increased when bees were fed dsRNA for the virus in the presence of virus in the colonies (Hunter et al., 2010). The ectoparasite Varroa mite is a major threat to the honey bee colony health and its management is a significant challenge. When honey bees ingest the mite-specific dsRNA that silences the calcium ion-binding protein known as calmodulin, the dsRNA is transmitted to the Varroa mite feeding on the hemolymph of the bees resulting in mite mortality (Garbian et al., 2012).
As with any new technology, it is important to consider the impact of RNAi on the environment and non-target organisms. Environmental risks and regulatory aspects of RNAi-based products have been reviewed in various reports (Liu et al., 2021; De Schutter et al., 2022; Christiaens et al., 2022). Microbial activity, UV radiation, and other environmental conditions degrade dsRNA and they are generally less stable in the environment, especially under the field conditions where they are used (Bachman et al., 2020). Studies showed that dsRNA degraded within two days in soil and 1-3 days in the aquatic environment (Dubelman et al., 2014; Fishcer et al., 2017). Chen et al. (2023) reported that while an RNAi-based biopesticide was highly effective against the 28-spotted ladybeetle (Henosepilachna vigintioctopunctata), a pest of solanaceous crops, it had no non-target effect on the predatory lady beetle Propylea japonica. Similarly, studies showed that the dsRNA developed for controlling Varroa mite were safe for honey bees (Tan et al., 2016; Vélez et al., 2016) and the monarch butterfly (Danaus plexxippus) whose calmodulin mRNA has a slight match to the Varroa-active dsRNA (Krishnan et al., 2021).
With regards to Ledprona, the US Environmental Protection Agency (EPA) found that it has minimal human and environmental risks due to low application rates, rapid microbial degradation in the environment, and physiological barriers and degradation mechanisms in mammals. EPA also gave Ledprona a “No Effect” determination according to the Endangered Species Act.
Environmental instability is one of the concerns for SIGS but formulation technology can address this problem. Instead of spraying naked dsRNA, formulating it with layered double hydroxide clay nanoparticles known as BioClay significantly extended the stability of dsRNA. Spraying dsRNA in BioClay provided protection against pepper mild mottle virus and cucumber mosaic virus at least for 20 days and dsRNA was detected on the leaves 30 days after application (Mitter et al., 2017). Similarly, spraying BioClay-formulated dsRNA 5 days before exposing to virus-containing green peach aphids (Myzus persicae) offered protection against the bean common mosaic virus in cowpea and benth (Nicotiana benthamiana) (Worrall et al., 2019). In a more recent study, BioClay-formulated dsRNA against gray mold (Botrytis cenerea) increased disease protection from 1 week to 3 weeks on leaves and 5 days to 10 days on fruit (Niño-Sánchez et al., 2022).
Arthropod pests and pathogens are resilient and rapidly evolving organisms and can develop resistance to RANi technology just like they develop to pesticides or transgenic crops. Whether it is HIGS or SIGS, avoiding heavy reliance on one tool and adopting integrated pest management (IPM) and resistance management strategies is crucial even when using RNAi. An IPM strategy that takes advantage of multiple tools will minimize the risk of resistance development while achieving desired pest suppression.
Additional resources:
Videos about RNAi: https://youtu.be/xDg6pu7HWz4 and https://youtu.be/cK-OGB1_ELE
References
Abdurakhmonov, I. Y., Z. T. Buriev, S. Saha, J. N. Jenkins, A. Abdukarimov and A. E. Pepper. 2014. Phytochorme RNAi enhances major fibre quality and agronomic traits of the cotton Gossypium hirsutum L. Nat. Comm. 5: 3062. https://doi.org/10.1038/ncomms4062.
Backman, P., J. Fischer, Z. Song, E. Urbanczyk-Wochniak and G. Watson. 2020. Environmental fate and dissipation of applied dsRNA in soil, aquatic systems, and plants. Front. Plant Sci. 11: 508351. https://doi.org/10.3389/fpls.2020.00021.
Bharathi, J. K., R. Anandan, L. K. Benjamin, S. Muneer, and M.A.S. Prakash. 2023. Recent trends and advances of RNA interference (RNAi) to improve agricultural crops and enhance their resilience to biotic and abiotic stresses. Plant Physiol. Biochem. 194: 600-618.
Chaudhary, D., A. S. Jeena, S. Gaur, R. Raj, S. Mishra, O. P. Gupta, and M. R. Meena. 2024. Advances in RNA interference for plant functional genomics: unveiling traits mechanisms, and future directions. Appl. Biochem. Biotechnol. https://doi.org/10.1007/s12010-023-04850-x.
Chen, S. X. Luo, S. Nanda, C. Yang, Z. Li, Y. Zhang, X. Zhou and H. Pan. 2023. RNAi-based biopesticides against 28-spotted ladybeetle Henosepilachna vigintioctopunctata does not harm the insect predator Propylea japonica. J. Agric. Food Chem. 71: 3373-3384.
Cheng, W., S. Yin, Y. Tu, H. Mei, Y. Wang and Y. Yang. 2020. SICAND1, encoding cullin-associated NEdd8-dissociated protein 1, regulates plant height, flowering time, seed germination, and root architecture in tomato. Plant Mol. Biol. 102: 537-551. https://doi.org/10.1007/s11103-020-00963-7.
Cheng, W., X.-S. Song, H.-P. Li, L.-H. Cao, K. Sun, X.-L. Qiu, Y.-B. Xu, P. Yang, T. Huang, J.-B. Zhang, B. Qu and Y.-C. Liao. 2015. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 13: 1335-1345. https://doi.org/10.1111/pbi.12352.
Christiaens, O., J. Sweet, T. Dzhambazova, I. Urru, G. Smagghe, K. Kostov and S. Arpaia. 2022. Implementation of RNAi-based arthropod pest control: environmental risks, potential for resistance and regulatory considerations. J. Pest Sci. 95: 1-15. https://doi.org/10.1007/s10340-021-01439-3.
De Schutter, K., C.N.T. Taning, L. Van Daele, E.J.M. Van Damme, P. Dubruel and G. Smagghe. 2022. RNAi-based biocontrol products: market status, regulatory aspects, and risk assessment. Front. Insect Sci. 1: 818037. https://doi.org/10.3389/finsc.2021.818037.
Dubelman, S., J. Fischer, F. Zapata, K. Huizinga, C. Jiang, J. Uffman, S. Levine and D. Carson. 2014. Environmental fate of double-stranded RNA in agricultural soils. PLoS One. https://doi.org/10.1371/journal.pone.0093155.
Dubrovna, O. V., S. I Mykhalska, and A. G. Komisarenko. 2023. Use of RNA interference technology for improving economically valuable traits of cereal crops. Cytology and Genetics 57: 587-610.
Dutta, T. K., P. K. Papolu, P. Banakar, D. Choudhary, A. Sirohi and U. Rao. 2015. Tomato transgenic plants expressing hairpin construct of a nematode protease gene conferred enhanced resistance to root-knot nematodes. Front. Microbiol. 6: 260. https://doi.org/10.3389/fmicb.2015.00260.
Enrique, R., F. Siciliano, M. A. Favaro, N. Gerhardt, R. Roeschlin, L. Rigano and M. R. Marano. 2011. Novel demonstration of RNAi in citrus reveals importance of citrus callose synthase in defence against Xanthomonas citri subsp. citri. Plant Biotehnol. J. 9: 394-407. https://doi.org/10.1111/j.1467-7652.2010.00555.x.
Eschen-Lippold, L., R. Ladgraf, U. Smolka, S. Schulze, M. Heilmann, I. Heilmann, G. Hause and S> ROsahl. 2012. Activation of defense against Phytophthora infestans in potato by down-regulation of syntaxin gene expression. The Ne Phytologist 193: 985-996. https://doi.org/10.1111/j.1469-8137.2011.04024.x.
Fischer, J. R., F. Zapata, S. Dubelman, G. M. Mueller, J. P. Uffman, C. Jiang, P. D. Jensen and S. L. Levine. 2017. Aquatic fate of a double-stranded RNA in a sediment-water system following an over-water application. Environ. Toxicol. Chem. 36: 727-734. https://doi.org/10.1002/etc.3585.
Fletcher, S. J., P. T. Reeves, B. T. Hoang, and N. Mitter. 2020. A perspective on RNAi-based biopesticides. Frontiers in Plant Science 11: 51. https://doi.org/10.3389/fpls.2020.00051.
Garbian, Y., E. Maori, H. Kalev, S. Shafir and I. Sela. 2012. Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PLoS Pathogens 8: e1003035. https://doi.org/10.1371/journal.ppat.1003035.
Guo, X., D. Chronis, C. M. De La Torre, J. Smeda, X. Wang and M. G. Mitchum. 2015. Enhanced resistance to sybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors. Plant Biotechnol. J. 13: 801-810. https://doi.org/10.1111/pbi.12313.
Head, G. P., M. W. Carroll, S. P. Evans, D. M. Rule, A. R. Willse, T. L. Clark, N. P. Storer, R. D. Flannagan, L. W. Samuel and L. J. Meinke. 2017. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: efficacy and resistance management. Pest Manag. Sci. 73: 1883-1899. https://doi.org/10.1002/ps.4554.
Hunter, W., J. Ellis, D. vanEngelsdorp, J. Hayes, D. Westervelt, E. Glick, M. Williams, I. Sela, E. Maori, J. Pettis, D. Cox-Foster and N. Paldi. 2010. Large-scale field application of RNAi technology reducing Israili Acute Paralysis Virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathogens 6: e1001160. https://doi.org/10.1371/journal.ppat.1001160.
Hunter, W. B. and W. M. Wintermantel. 2021. Optimizing efficient RNAi-mediated control of hemipteran pests (psyllids, leafhoppers, whitefly): modified pyrimidines in drRNA triggers. Plants 10: 1782. https://doi.org/10.3390/plants10091782.
Jain, R. G., S. J. Fletcher, N. Manzie, K. E. Robinson, P. Li, E. Lu, C. A. Brosnan, Z. P. Xu and N. Mitter. 2022. Foliar application of clay-delivered RNA interference for whitefly control. Nature Plants 8: 535-548.
Jiang, C.-J., M. Shimono, S. Maeda, H. Inoue, M. Mori, M. Hasegawa, S. Sugano and H. Takatsuji. 2009. Suppression of the rice fatty-acid desaturase gene OsSSI2 enhances resistance to blast and leaf blight diseases in rice. Mol. Reprod. Dev. 22: 820-829. https://doi.org/10.1094/MPMI-22-7-0820.
Jiao, C., J. Sun. and Y. Wei. 2024. SlWRKY31 enhances chilling tolerance by interacting with SlSIZ1 in tomato fruit. Postharvest Biol. Technol. 207: 112631. https://doi.org/10.1016/j.postharvbio.2023.112631.
Kim, H. J., M. J. Kim, J. H. Pak, H. H. Im, D. H. Lee, K. H. Ki, and Y. S. Chung. 2016. RNAi-mediated soybean mosaic virus (SMV) resistance of a Korena soybean cultivar. Plant Biotechnol. Reports 10: 257-267. https://doi.org/10.1007/s11816-016-0402-y.
Koch, A., D. Biedenkopf, A. Furch, L. Weber, O. Rossbach, E. Abdellatef, L. Linicus, J. Johannsmeier, L. Jelonek, A. Goesmann, V. Cardoza, J. McMillan, T. Mentzel and K.-H. Kogel. 2016. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathogens 12: e1005901. https://doi.org/10.1371/journal.ppat.1005901.
Krishnan, N., M. J. Hall, R. L. Hellmich, J. R. Coats and S. P. Bradbury. 2021. Evaluating toxicity of Varroa mite (Varroa destructor)-active dsRNA to monarch butterfly (Danaus Plexippus) larvae. PLoS One 16: e0251884. https://doi.org/10.1371/journal.pone.0251884.
Li, H. R. Guan, H. Guo and X. Miao. 2015. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant, Cell & Environment 38: 2277-2285. https://doi.org/10.1111/pce.12546.
Liu, S., S. Geng, A. Li, Y. Mao and L. Mao. 2021. RNAi technology for plant protection and its application in wheat. aBIOTECH 2: 365-374. https://doi.org/10.1007/s42994-021-00036-3.
Mao, Y. B., W. J. Cai, J. W. Wang, G. J. Hong, X. Y. Tao, L. J. Wang and X. Y. Chen. 2007. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotehnol. 25: 1307-1313. https://doi.org/10.1038/nbt1352.
Mao, Y. B., X. Y. Tao, X. Y. Xue, L. J. Wang and X. Y. Chen. 2011. Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Trans. Res. 20: 665-673. https://doi.org/10.1007/s11248-010-9450-1.
Mitter, N., E. A. Worrall, K. E. Robinson, P. Li, R. G. Jain, C. Taochy, S. J. Fletcher, B. J. Carroll, G. Q. Lu and Z. P. Xu. 2017. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 3: 16207. https://doi.org/10.1038/nplants.2016.207.
Niño-Sánchez, J., P. T. Sambasivam, A. Sawyer, R. Hamby, A. Chen, E. Czislowski, P. Li, N. Manzie, D. M. Gardiner, R. Ford, Z. P. Xu, N. Mitter and H. Jin. BioClayTM prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integrative Pl. Biol. 64: 2187-2198. https://doi.org/10.1111/jipb.13353.
Qiao, F., Q. Yang, C. L. Wang, Y. L. Fan, X. F. Wu, and K. J. Zhao. 2007. Modification of plant
height via RNAi suppression of OsGA20ox2 gene in rice. Euphytica 158: 35–45.
https://doi.org/10.1007./s10681-007-9422-6.
Rodrigues, T., K. Sridharan, B. Manley, D. Cunningham and K. Narva. 2021. Development of dsRNA as a sustainable bioinsecticide: from laboratory to field. In: Rauzan BM and Lorsbach BA, editors. Crop protection Products for Sustainable Agriculture, ACS Symposium Series. 1390. Washington, DC: ACS Publications, p. 65–82.
Tan J., S. L. Levine, P. M. Bachman, P. D. Jensen, G. M. Mueller, J. P. Uffman, C. Meng, Z. Song, K. B. Richards and M. H. Beevers. 2016. No Impact of DvSnf7 RNA on Honey Bee (Apis Mellifera L.) Adults and Larvae in Dietary Feeding Tests. Environ. Toxicol. Chem. 35: 287–294. https://doi.org/10.1002/etc.3075.
Vélez, A. M., J. Jurzenski, N. Matz, X. Zhou, H. Wang, M. Ellis and B. D. Siegfried. 2016. Developing an in Vivo Toxicity Assay for RNAi Risk Assessment in Honey Bees, Apis Mellifera L. Chemosphere 144: 1083–1090. https://doi.org/10.1016/j.chemosphere.2015.09.068.
Worrall, E. A., A. Bravo-Cazar, A. T. Nilon, S. J. Fletcher, K. E. Robinson, J. P. Carr and N. Mitter. 2019. Exogenous application of RNAi-induced double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 10: 265. https://doi.org/10.3389/fpls.2019.00265.
Yang, J., Y. Zhang, J. Zhao, Y. Gao, Z. Liu, P. Zhang, R. Fan, S. Xing and X. Zhou. 2023. Target gene selection for RNAi-based biopesticides against the hawthorn spider mite, Amphitetranychus viennensis (Acari: Tetranychidae). Pest Manag. Sci. 79: 2482-2492.
Zha, W., X. Peng, R. Chen, B. Du, L. Zhu, and G. He. 2011. Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens. PLoS One 6: e20504. https://doi.org/10.1371/journal.pone.0020504.
Zhao, K., F. Zhang, Y. Yang, Y. Ma, Y. Liu, H. Li, and Z. Zhang. 2016. Modification of plant height via RNAi suppression of MdGA20-ox gene expression in apple. J. Am. Soc. Hort. Sci. 141: 242-248. https://doi.org/10.21273/JASHS.141.3.242.